| Dengue virus Envelop protein |
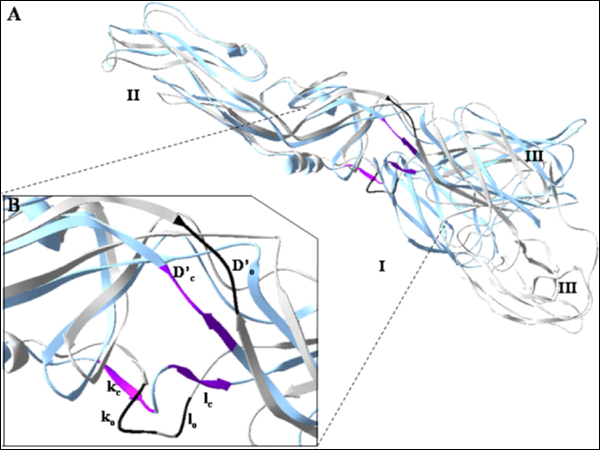
Figure 1. Pre-fusion (PDB code 1oke) and post-fusion (PDB code 1ok8) conformations of Dengue virus E
protein and the ligand-binding pocket for virtual screening
Limited structural information of drug targets, cellular toxicity possessed by lead compounds,
and large amounts of potential leads are the major issues facing the design-oriented approach
of discovering new leads. In an attempt to tackle these issues, we have developed a process of
virtual screening based on the observation that conformational rearrangements of the dengue virus
envelope protein are essential for the mediation of viral entry into host cells via membrane fusion.
Screening was based solely on the structural information of the Dengue virus envelope protein
and was focused on a target site that is presumably important for the conformational rearrangements
necessary for viral entry. To circumvent the issue of lead compound toxicity, we performed screening
based on molecular docking using structural databases of medical compounds. To enhance the identification
of hits, we further categorized and selected candidates according to their novel structural
characteristics. Finally, the selected candidates were subjected to a biological validation assay
to assess inhibition of Dengue virus propagation in mammalian host cells using a plaque formation
assay.
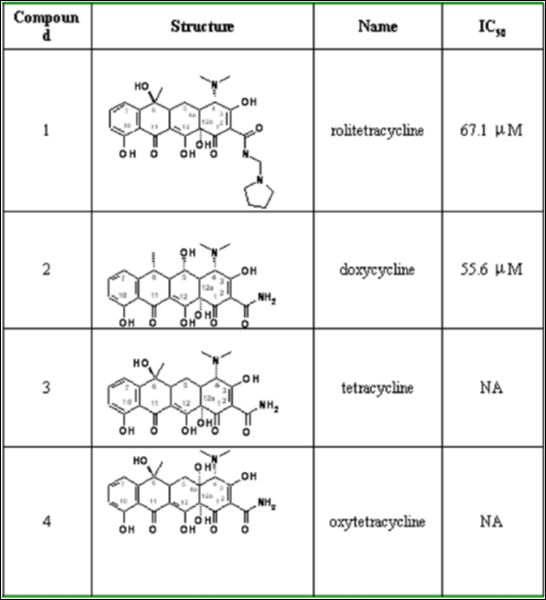
Figure 2. Chemical Structures and IC50s for the tetracycline derivatives.
Among the 10 compounds examined, rolitetracycline and doxycycline significantly inhibited plaque
formation, demonstrating their inhibitory effect on dengue virus propagation. Both compounds were
tetracycline derivatives with IC50s estimated to be 67.1 £gM and 55.6 £gM, respectively. Their docked
conformations displayed common hydrophobic interactions with critical residues that affected membrane
fusion during viral entry. These interactions will therefore position the tetracyclic ring moieties of
both inhibitors to bind firmly to the target and, subsequently, disrupt conformational rearrangement
and block viral entry. This process can be applied to other drug targets in which conformational
rearrangement is critical to function.
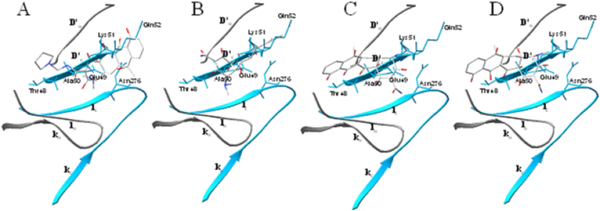
Figure 3. Docked conformations of the four tetracycline derivatives related to residues
48¡V52 in the BOG binding site in the pre-fusion (gray) and post-fusion (blue) states.
Reference:
J.-M. Yang, Y.-F. Chen, Y.-Y. Tu, K.-R. Yen, and Y.-L. Yang*, ¡§Combinatorial
computation approaches identifying tetracycline derivates as flaviviruses inhibitors,¡¨
PLoS ONE, pp. e428.1- e428.12, 2007.
| |
