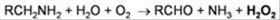
ECAO is a copper-containing amine oxidase for industrial purpose. On our research
cooperating with
Chjun-Jye Yuan, we have known that the enzyme catalyze the metabolic oxidative
deamination of primary amines to the corresponding aldehydes, with concurrent
production of hydrogen peroxide and ammonia. This reaction proceeds through a
transamination mechanism mediated by an active site 2,4,5-trihydroxyphenylalanine
quinone (TPQ) cofactor, formed from the post-translational self-processing of tyrosine.
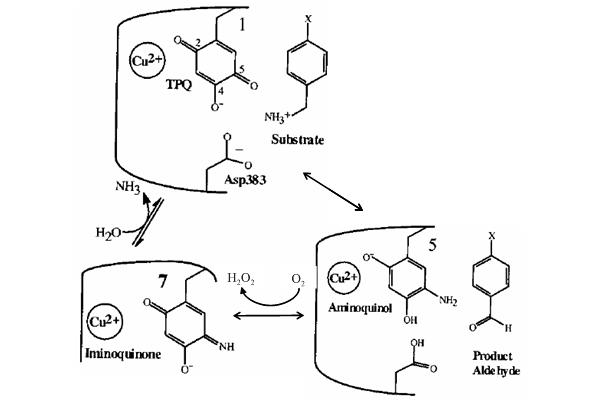 |
| Figure. 1 The catalytic mechanism of ECAO. (from Science, 286, 1724-1728, 1999) |
In Figure 1, During the interaction, the -NH2 group of the substrate was substituted for the O-5 of TPQ. We have docked the substrate to two cavities by GEMDOCK:
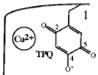
| 1. Oxidative form |
 |
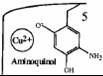
| 2. Reductive form |
 |
The two cavities were identical except the form of TPQ. However the docking results were far apart.
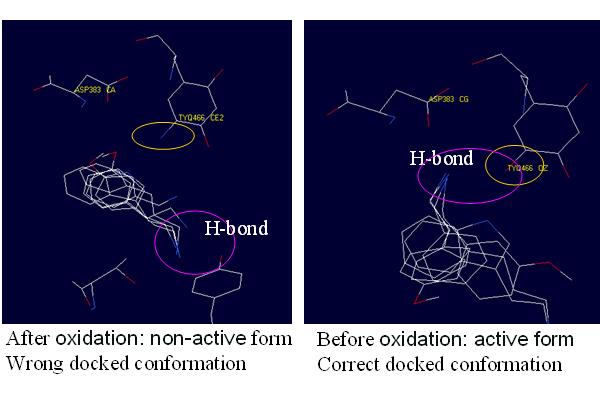 |
| Figure 2. The docked conformation of the substrate in two different binding sites. |
When we docked the substrate to the first cavity, the -NH2 group of the docked
conformation directed to the residue TPQ and ASP383 as well as the crystal.
When we docked the ligand to the second cavity, the -NH2 group of the docked
conformation directed to TYR opposite TPQ and ASP383. The results were both
expected in two cases, because the -NH2 group of the ligand tended to form hydrogen
bonds with =O and -OH of the binding site. Therefore we considered that GEMDOCK
is very sensitive to the sidechain of the binding site. Even only slight variation
might lead to a great effect and it is very important to select a prefferred
cavity for docking.
|
